Acetaminophen overdose: Science and Resources
Acetaminophen overdose: the numbersThe physiology of acetaminophen overdose
Mechanism of Action
Acetaminophen overdose
When time is liver, timing is critical
Liver function tests
Continuing therapy after 21-hour regimen
Products containing acetaminophen
Resources
Acetaminophen overdose: the numbers
In the U.S. more than 28 billion doses of medicines containing acetaminophen are purchased each year.1
- From 1998 to 2003, acetaminophen was the leading cause of acute liver failure (ALF) in the United States, with 48% of acetaminophen-related cases (131 of 275) associated with accidental overdose
- A 2007 CDC population-based report estimates that, nationally, there are 1,600 cases of ALF each year (all causes). Acetaminophen-related ALF was the most common etiology
The physiology of acetaminophen overdose2
Acetaminophen Metabolism
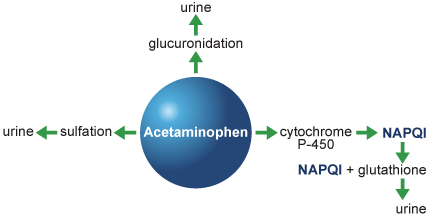
- Acetaminophen is metabolized through 3 different pathways:
- 42% to 67% undergoes glucuronidation and is excreted in urine
- 26% to 36% undergoes sulfation and is excreted in urine
- 5% to 8% passes through the cytochrome P-450 pathway producing a potentially hepatotoxic metabolite, N-acetyl-p-benzoquinone imine (NAPQI)
- In normal dosing, NAPQI is conjugated with the antioxidant, glutathione, and excreted in urine
Mechanism of Action
Overdose depletes the available glutathione and unbound NAPQI destroys liver cells.2
IV Acetadote protects liver cells in 2 ways:
- It helps replenish glutathione
- It binds to and removes NAPQI

Acetaminophen overdose
- The maximum adult therapeutic dose of acetaminophen is 4 grams/24 hours3
- The maximum dose for children less than 12 years is 75 mg/kg4
- Acute overdose is defined as ingestion of ≥ 150 mg/kg or 12 grams/24 hours4
Four stages of acetaminophen overdose5
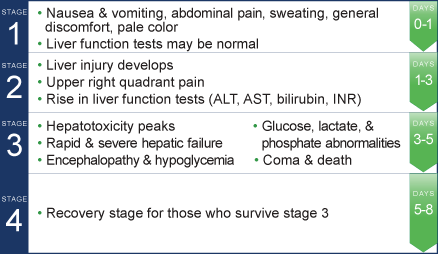
When time is liver, timing is critical
Treatment Recommendations
The following recommendations are related to acute acetaminophen ingestion. For recommendations related to repeated supratherapeutic exposure see Dosage and Administration.6
- 1. Assess the history and timing of acetaminophen ingestion as an overdose.
- The reported history of the quantity of acetaminophen ingested as an overdose is often inaccurate and is not a reliable guide to therapy.
- 2. Obtain the following laboratory tests to monitor hepatic and renal function and electrolyte and fluid balance: aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, international normalized ratio (INR), creatinine, blood urea nitrogen (BUN), blood glucose, and electrolytes.
- 3. Obtain a plasma or serum sample to assay for acetaminophen concentration at least 4 hours after ingestion. Acetaminophen concentrations obtained earlier than 4 hours post-ingestion may be misleading as they may not represent maximum acetaminophen concentrations.
- 4. If the time of acute acetaminophen ingestion is unknown:
- Administer a loading dose of ACETADOTE immediately.
- Obtain an acetaminophen concentration to determine need for continued treatment.
- 5. If the acetaminophen concentration cannot be obtained (or is unavailable or uninterpretable) within the 8- hour time interval after acetaminophen ingestion or there is clinical evidence of acetaminophen toxicity:
- Administer a loading dose of ACETADOTE immediately and continue treatment for a total of three doses over 21 hours.
- 6. If the patient presents more than 8 hours after ingestion and the time of acute acetaminophen ingestion is known:
- Administer a loading dose of ACETADOTE immediately.
- Obtain an acetaminophen concentration to determine need for continued treatment.
- 7. If the patient presents less than 8 hours after ingestion and the time of acute acetaminophen ingestion is known and the acetaminophen concentration is known:
- Use the Rumack-Matthew nomogram to determine whether or not to initiate treatment with ACETADOTE.
Click Here for Full Prescribing Information
Liver function tests
Liver function tests can help determine hepatic injury

Other helpful tests include:
- Bilirubin
- INR
Continuing therapy after 21-hour regimen
In cases of suspected massive overdose, or with concomitant ingestion of other substances, or in patients with preexisting liver disease; the absorption and/or the half-life of acetaminophen may be prolonged. In such cases, consideration should be given to the need for continued treatment with ACETADOTE beyond a total of three separate doses over a 21-hour infusion period.
Acetaminophen levels and ALT/AST and INR should be checked after the last maintenance dose. If acetaminophen levels are still detectable, or if the ALT/AST are still increasing or the INR remains elevated; the maintenance doses should be continued and the treating physician should contact a US regional poison center at 1-800-222-1222, alternatively, a “special health professional assistance line for acetaminophen overdose” at 1-800-525-6115 for assistance with dosing recommendations, or 1-877-484-2700 for additional information.
Click Here for Full Prescribing Information
Comprehensive List of OTC and prescription products
containing acetaminophen
For the most up-to-date information please visit the National Library of Medicine.
Resources
National Poison Control Center
1-800-222-1222http://www.nlm.nih.gov/medlineplus/ency/article/002724.htm
American Association of Poison Control Centers (AAPCC)
www.aapcc.orgCDC
http://www.nlm.nih.gov/medlineplus/ency/article/002598.htme-Medicine: Acetaminophen Toxicity
http://emedicine.medscape.com/article/820200-overviewPediatric Toxicity
http://emedicine.medscape.com/article/1008683-overviewSelected Articles
- Acetaminophen Poisoning. Merck Manuals Online Medical Library for Healthcare Professionals. Available at: http://www.merckmanuals.com/professional/print/sec22/ch346/ch346b.html.
- Anderson BJ, Holford NH, Armishaw JC, Aicken R. Predicting concentrations in children presenting with acetaminophen overdose. J Pediatr. 1999;135:290-295.
- Bizovi KE, Aks SE, Paloucek F, Gross R, Keys N, Rivas J. Late increase in acetaminophen concentration after overdose of Tylenol Extended Relief. Ann Emerg Med. 1996;28:549-551.
- Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102:2459-2463.
- Heard KJ, Green JL, James LP, et al. Acetaminophen-cysteine adducts during therapeutic dosing and following overdose. BMC Gastroenterol. 2011. Available at: http://www.biomedcentral.com/content/pdf/1471-230X-11-20.pdf. Accessed May 10, 2010.
- Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity-a clinically relevant model to test the efficacy of natural products. Life Sci. 2011. [Epub ahead of print]
- Lee W. Drug-induced hepatotoxicity. New Eng J Med. 2003; 349:474-485. Available at: http://gastro.dom.uab.edu/Fellow_Articles/fellowsreadinglisthep/drug%20hepatotoxicity.pdf
- Mayhew M. Acetaminophen toxicity. J Nurs Practitioners. 2007;3:186-188. Available at: http://www.medscape.com/viewarticle/557074.
- Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics. 1975;55:871-876.
- Stumpf JL, Skyles AJ, Alaniz C, Erickson SR. Knowledge of appropriate acetaminophen doses and potential toxicities in an adult clinic population. J Am Pharm Assoc. 2007;47:35-41.
INDICATION
Acetadote is an antidote for acetaminophen overdose indicated to prevent or lessen hepatic injury after ingestion of a potentially hepatotoxic quantity of acetaminophen in patients with an acute ingestion or from repeated supratherapeutic ingestion.
IMPORTANT SAFETY INFORMATION
ADVERSE EVENTS
In the literature, the most frequently reported adverse reactions attributed to intravenous acetylcysteine administration were rash, urticaria and pruritus. The frequency of adverse reactions have been reported to be between 0.2% and 21%, and they most commonly occur during the initial loading dose of acetylcysteine.
View full Prescribing Information
References
-
FDA. Drugs, Drug Safety and Risk Management Committee. Acetaminophen overdose and liver injury – background and options for reducing injury. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/UCM164897.pdf. Accessed June 1, 2011.
-
Background package on acetaminophen. Acetaminophen metabolism. McNeil Pharmaceuticals. Available at: http://www.fda.gov/ohrms/dockets/ac/02/briefing/3882B1_13_McNeil-Acetaminophen.htm#_Toc18717570. Accessed May 24, 2011.
-
Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364-1372.
-
Farrell SE. Toxicity, acetaminophen: Background. E-medicine. Available at : http://emedicine.medscape.com/article/820200-overview. Accessed November 14, 2016.
-
Acetaminophen poisoning. Merck Manual. Available at: http://www.merckmanuals.com/professional/sec21/ch326/ch326c.html. Accessed May 24, 2011. Accessed May 24, 2011.
-
Acetatode Prescribing Information. Cumberland Pharmaceuticals Inc. 2016.
-
Rumack BH, Peterson RC, Kock GG, et al. Acetaminophen overdose: 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med. 1981;141:380-385.
-
Farrell SE. Toxicity, acetaminophen: differential diagnosis and workup. E-medicine. Available at : http://emedicine.medscape.com/article/820200-workup. Accessed May 24, 2011.
